-
- PCB TYPE
- PRINTED CIRCUIT BOARD PROTOTYPE ALUMINUM PRINTED CIRCUIT BOARD R&F PCB FPC HIGH FREQUENCY PCB HIGH-TG PCB HEAVY COPPER PCB HDI PCB PCB FOR LIGHTING METAL CORE PCB
time:Dec 22. 2025, 13:11:05
The healthcare industry stands at the forefront of technological innovation, with electronic devices playing an increasingly pivotal role in diagnosis, treatment, and patient care. From portable diagnostic tools to implantable medical devices, these innovations rely on components that meet the most stringent standards of precision, reliability, and biocompatibility. Among these critical components, flexible printed circuit boards (FPCBs) have become indispensable, and Medical Grade FPCB Assembly Solutions emerge as a cornerstone to ensure these boards meet the unique demands of the healthcare sector. Unlike standard FPCB assembly services, Medical Grade FPCB Assembly Solutions are engineered to adhere to rigorous regulatory frameworks and clinical safety requirements, making them essential for advancing life-saving medical technologies.
Medical devices operate in highly sensitive environments, where even the smallest component failure can have catastrophic consequences for patient safety. This reality defines the core imperatives of Medical Grade FPCB Assembly Solutions: uncompromising compliance, exceptional reliability, and strict biocompatibility. Regulatory bodies such as the FDA (U.S.), CE (EU), and ISO (international) have established rigorous standards for medical electronic components, and Medical Grade FPCB Assembly Solutions must fully align with these guidelines. This includes compliance with ISO 13485, the international standard for medical device quality management systems, which governs every stage of the assembly process from material selection to final testing.
Beyond compliance, Medical Grade FPCB Assembly Solutions prioritize reliability that exceeds industrial or consumer electronics standards. Medical devices often operate continuously for extended periods—sometimes inside the human body—and FPCBs must withstand harsh conditions such as body fluids, temperature fluctuations, and mechanical stress without degradation. Biocompatibility is another non-negotiable requirement: materials used in Medical Grade FPCB Assembly Solutions must be non-toxic, non-irritating, and resistant to corrosion from biological environments, ensuring they do not pose risks to patients.
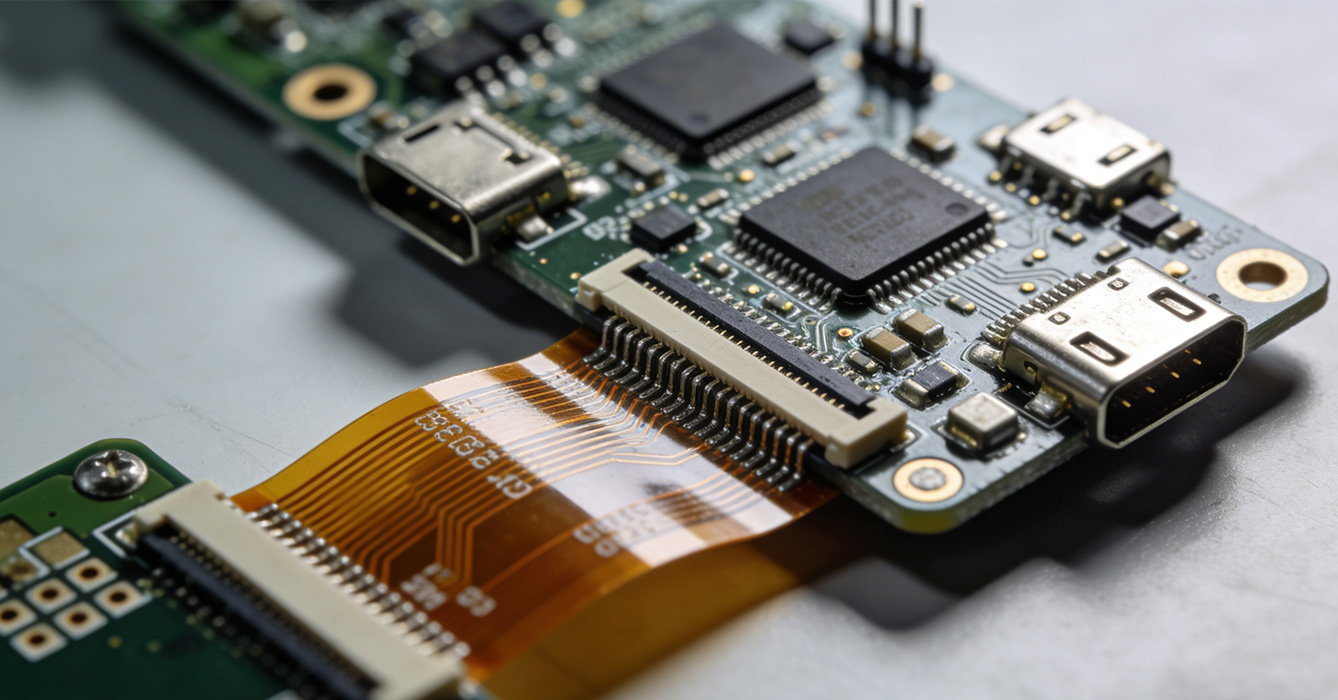
Medical Grade FPCB Assembly Solutions power a wide range of life-changing medical devices, each leveraging the flexibility and miniaturization advantages of FPCBs while adhering to medical-grade standards. One of the most critical applications is in implantable medical devices, such as pacemakers, defibrillators, and neurostimulators. These devices require ultra-compact, lightweight FPCBs that can fit within the body’s confined spaces, and Medical Grade FPCB Assembly Solutions ensure the boards are assembled with microscopic precision to avoid electrical malfunctions that could endanger patients.
Portable and wearable diagnostic devices also rely heavily on Medical Grade FPCB Assembly Solutions. Devices like blood glucose monitors, heart rate trackers, and portable ultrasound machines use FPCBs to achieve sleek, ergonomic designs that enhance patient comfort and usability. The assembly solutions for these devices must ensure accurate signal transmission for reliable diagnostic results, as well as durability to withstand repeated use in clinical and home settings. Additionally, in vitro diagnostic (IVD) equipment, such as automated analyzers and molecular testing devices, uses medical-grade FPCB assemblies to support high-throughput, precise operations that are critical for timely disease detection.

The integrity of Medical Grade FPCB Assembly Solutions hinges on robust quality control (QC) processes that leave no room for error. Leading providers implement QC measures at every stage of the assembly workflow, starting with material inspection. All substrates, components, and adhesives used in medical-grade assembly are rigorously tested for biocompatibility, chemical resistance, and electrical performance to ensure they meet regulatory standards. During assembly, precision equipment—such as automated optical inspection (AOI) and x-ray inspection systems—is used to detect even the smallest defects, such as solder bridges or component misalignment, which could compromise device functionality.
Post-assembly testing is equally critical. Medical Grade FPCB Assembly Solutions include comprehensive electrical testing, such as continuity testing and functional testing, to verify that the assembled boards perform as intended under simulated clinical conditions. Some applications also require environmental testing, where FPCBs are exposed to extreme temperatures, humidity, and chemical agents to validate their durability. Additionally, traceability is a key QC component: reputable providers maintain detailed records of every assembly batch, allowing for full traceability from raw materials to the final medical device, which is essential for regulatory audits and patient safety.
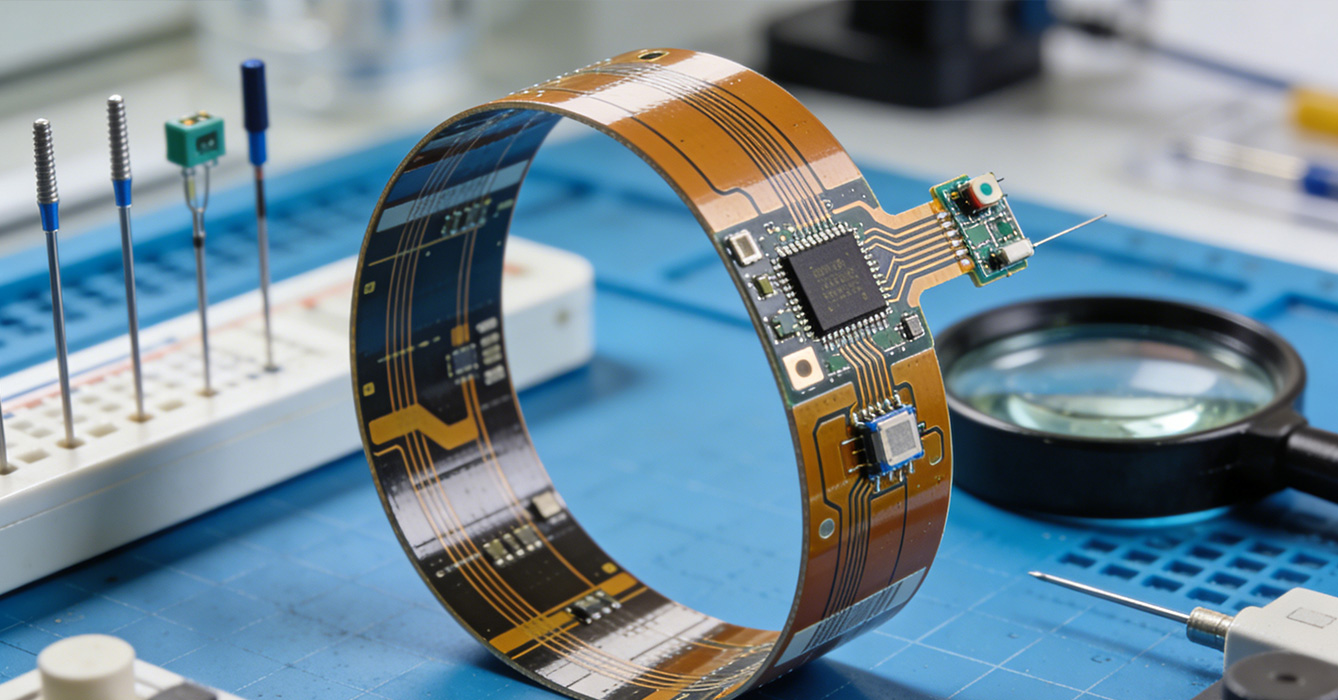
Choosing a qualified provider for Medical Grade FPCB Assembly Solutions is a critical decision that directly impacts patient safety and regulatory compliance. When evaluating potential partners, the first priority is verifying their regulatory credentials—ensure the provider is ISO 13485 certified and has a proven track record of complying with FDA, CE, and other relevant regulatory requirements. Experience in the medical sector is also essential: look for providers with expertise in assembling FPCBs for your specific type of medical device, as this ensures they understand the unique challenges and requirements of your application.
Collaboration and communication are also key factors. A reliable provider will work closely with your engineering team to optimize FPCB designs for medical-grade assembly, offering guidance on material selection, design for manufacturability (DFM), and regulatory compliance. They should also provide transparent reporting throughout the assembly process, including detailed QC results and traceability documentation. Finally, assess the provider’s capacity to scale production, as medical device manufacturers often need to transition from prototyping to large-scale production while maintaining consistent quality.
In conclusion, Medical Grade FPCB Assembly Solutions are essential for advancing modern healthcare, enabling the development of safe, reliable, and innovative medical devices that improve patient outcomes. By prioritizing compliance, precision, and quality control, these solutions meet the unique demands of the medical industry and support regulatory adherence. As healthcare technology continues to evolve—with trends such as miniaturization and wearable medical devices gaining momentum—the role of Medical Grade FPCB Assembly Solutions will only become more critical. Choosing the right provider, one with regulatory expertise, rigorous QC processes, and medical industry experience, is the key to unlocking the full potential of FPCB technology in life-saving medical applications.

Got project ready to assembly? Contact us: info@apollopcb.com



We're not around but we still want to hear from you! Leave us a note:

Leave Message to APOLLOPCB
